Politics and Policy
Latest News
Latest Videos

Podcasts
CME Content
More News

Pharmacy benefit managers aren't getting mentioned on the campaign trail, but there are some signs Congress could enact reform legislation after the Nov. 5 election.

Reproductive rights and abortion: The hottest healthcare issue

Projections calculated for AARP show that 8.4% of Part D enrollees will benefit from the Inflation Reduction Act's $2,000 cap on out-of-pocket costs that goes into effect in 2025.

Perceptions of affordability don't show an advantage to Medicare Advantage, say former and current Commonwealth Fund leaders.

Congressional Budget Office analysts say that Medicaid unwinding, immigration and scheduled end of ACA premium subsidies are factors in the increase.

Patients with PTSD in the clinical trials of midomafetamine were able to guess whether they received treatment or placebo. Regulators and advisory committee members said this could have impacted efficacy results. FDA’s decision is expected by Aug. 11, 2024.

mRESVIA will be available for the 2024/2025 RSV season as a pre-filled, ready to use syringe.
Zolbetuximab is first-in-class monoclonal antibody. The FDA has assigned an action date of Nov. 9, 2024.

FDA officials have asked for additional analyses on the efficacy of Dupixent in the two pivotal trials. The revised target action date is Sept. 27, 2024.
Breyanzi is a CAR T-cell therapy now approved for four subtypes of non-Hodgkin lymphoma.
Zanidatamab is bispecific antibody that targets HER2 in patients with metastatic biliary tract cancer. The FDA has set an action date of Nov. 29, 2024.
The FDA has set a Prescription Drug User Fee Act date of Nov. 27, 2024, to treat patients with PIK3CA-mutated HR-positive, HER2 negative metastatic breast cancer.
Bkemv is a monoclonal antibody that is approved to treat two rare conditions that break down red blood cells.
Verastem is studying the combination of avutometinib and defactinib to treat low-grade serous ovarian cancer in patients with KRAS-mutations. The company plans to complete the new drug application in the second half of this year.
Yesafili and Opuviz are approved to treat patients with neovascular (wet) age-related macular degeneration, macular edema following retinal vein occlusion, diabetic macular edema and diabetic retinopathy.
If approved, tabelecleucel would be the first therapy specifically to treat Epstein-Barr virus related post-transplant lymphoproliferative disease.
Tarlatamab — now with the brand name Imdelltra — is the first approved bispecific antibody for a solid tumor.

Breyanzi is now included in the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology as a recommendation for third-line therapy for relapsed or refractory follicular lymphoma.
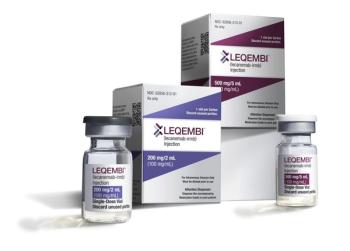
If approved, the Leqembi autoinjector could be used to administer the treatment for Alzheimer’s disease at home or at medical facilities.

Regulators are now working to complete their review of the BLA for mRNA-1345 by the end of May 2024. mRNA-1345 to be reviewed at the CDC's Advisory Committee on Immunization Practices in June 2024.

If approved, midomafetamine would be the first psychedelic-assisted therapy approved for any mental health condition. The advisory committee meeting is June 4, 2024.

Zenocutuzumab is a new type of bispecific antibody that target both HER2 and HER3 proteins to inhibit NRG1 binding and blocking the mechanism for tumor survival. The FDA’s target date is in December 2024.

The Peripheral and Central Nervous System Drugs Advisory Committee will meet on Monday, June 10, 2024, to discuss the phase 3 trial of Lilly’s donanemab to treat patients with early symptomatic Alzheimer’s disease.

Myhibbin is a ready-to-use mycophenolate mofetil oral suspension to prevent rejection of kidney, heart and liver transplants.

Bristol Myers Squibb is seeking approval of the subcutaneous formulation for all previous indications of Opdivo. The FDA has assigned a goal date of Dec. 29, 2024.