Biosimilars
Latest News
Latest Videos

CME Content
More News

Sandoz’s Tyruko (natalizumab), the only FDA approved natalizumab biosimilar for adults with relapsing multiple sclerosis and Crohn’s disease, is now available by prescription in the United States under a restricted-distribution program because of the drug’s risk of progressive multifocal leukoencephalopathy.

The FDA has approved Poherdy as the first interchangeable biosimilar to Perjeta, expanding treatment options for adults with HER2-positive breast cancer across both early-stage and metastatic settings.

Increased adoption of biosimilars requires regulatory changes, educational efforts, and PBM reimbursement policies that enable fair competition, argues Dr. Benjamin Rome in a new paper.

Biosimilars enhance arthritis treatment access, with rising adoption among women and long-term patients, revealing key prescribing patterns in Turkey.

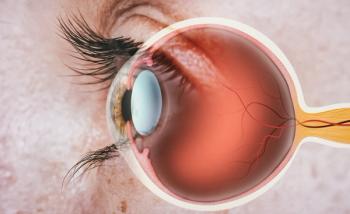
Vascular endothelial growth factor (VEGF) inhibitor, Eydenzelt (aflibercept-boav), is now approved to treat retinal disease marked by excessive blood vessel growth in adults.
A recent study finds that despite biosimilar use rising to 60% to 86% for three cancer drugs, predicted savings don’t match those that would be achieved from a site-neutral reimbursement strategy.

Amneal submits a BLA for a new Xolair biosimilar, promising affordable treatment options for asthma and allergies, enhancing patient access and competition.
The FDA's groundbreaking decision to waive clinical efficacy studies for a monoclonal antibody biosimilar revolutionizes drug approval, reducing costs and enhancing patient access.
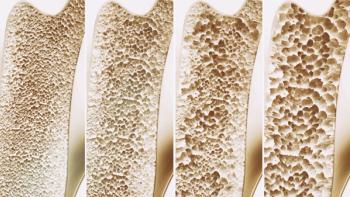
Bildyos and Bilprevda have the same indications as the reference products Prolia and Xgeva and are expected to be available later this year.

The biosimilar will be available to any licensed specialty pharmacy in the United States at a 95% discount from Stelara, starting January 1, 2026.

Prefilled syringes of Imuldosa now have the lowest wholesaler acquisition cost among all branded ustekinumab biosimilars, according to a news release.

Specialty drug spending rose 9.6% in 2024, but Humira biosimilar adoption is helping to slow cost increases.

Originally approved earlier this year for multiple inflammatory conditions, including rheumatoid arthritis, giant cell arteritis, juvenile idiopathic arthritis and COVID-19-related inflammation, Avtozma now aligns fully with the FDA-approved indications of its biosimilar, Actemra.

This biosimilars market report focuses on rising market complexity and recent activity, including two new approvals and four launches in Q2.

Since its approval in 2002, Humira has become the world’s top-selling drug—largely due to long-standing market exclusivity and rising prices in the U.S.
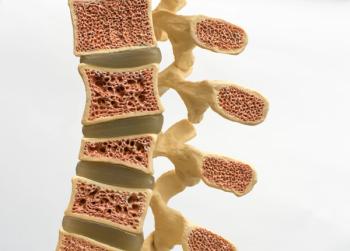
Wyost and Jubbonti are interchangeable with Xgeva/Prolia and are approved for all of the same indications in bone cancer and osteoporosis.

Respondents see the glucagon-like peptide 1 (GLP-1) drugs as a major advance. They also have concerns about whether insurers can afford to cover them and about wasteful prescriptions.
It has been 10 years since the first biosimilar was approved by the FDA. The grades on how the market for biosimilars has developed are mixed.

The second part of our conversation with Craig Burton, MBA, executive director of the Biosimilars Council, a trade and lobbying group for the biosimilars industry.

We spoke recently with Craig Burton, MBA, executive director of the Biosimilars Council, a trade and lobbying group for the biosimilars industry.

Using a single PBM as the sole distribution channel is unprecedented.

Will the Stelara biosimilars be a reprise of the Humira biosimilars in 2023 and be slow to catch on?

The report also discusses the spurt of Stelera biosimilar approvals in late 2024.
In 2023, there was lower net spending and prices on adalimumab, which was likely because of rebates from AbbVie, Humira’s manufacturer, a new analysis finds.