
The Institute for Clinical and Economic Review’s fair access report found that it was difficult to determine how well policies translate into real-world access and affordability for patients.

The Institute for Clinical and Economic Review’s fair access report found that it was difficult to determine how well policies translate into real-world access and affordability for patients.

The Institute for Clinical and Economic Review’s fair access report found that for the drugs assessed, formularies provided fair access, but it was difficult to determine how well that translates into real-world access and affordability for patients.

Sarepta Therapeutics plans to submit an application for full approval of Elevidys to treat all ages of patients with Duchenne muscular dystrophy. Company officials said the FDA is open to evaluating the application based on the totality of the evidence.
Almost 80% of patients in an extension study maintained clear or almost clear skin, and 90% of patients saw continued improvements in itch.
The Humira biosimilar Yuflyma will be available through CarePartners specialty pharmacy business, which has 10 million plan members.

Based on reports from post-marketing experience, Spravato’s warnings and precautions section now includes the risk of respiratory depression.
Mirikizumab — now with the brand name — Omvoh is the first therapy that targets interleukin-23p19, which plays a role in inflammation. It has a list price of $9,593 per month for the IV formulation and $10,360 per subcutaneous administration.
In new data, 60% of the patients with early stage Alzheimer’s disease and who have low levels of the protein tau achieved cognitive improvement when treated with Leqembi.

RP-L201 (marnetegragene autotemcel) is a one-time gene therapy that delivers a functional copy of the ITGB2 gene, which provides instructions for immune response to infections. The Prescription Drug User Fee Act (PDUFA) target action date is March 31, 2024.

New results demonstrate seladelpar can reduce alkaline phosphatase and itching in patients with primary biliary cholangitis, a chronic liver disease.

Celltrion’s Zymfentra is subcutaneous version of infliximab.
Zymfentra is a subcutaneous version of infliximab.

Researchers at the Yale School of Medicine have invented a new delivery technology that has the potential to create one-time treatments for a range of neurological disorders.
Research continues to develop new therapies for rare cancers and to provide options that allow for fewer toxicities. At the same, however, more new drugs are launching with high price tags.

Using an AI platform, Magellan Health was able to better support providers in prescribing behavioral health medications and addressing medication problems, which reduced pharmacy costs and increased adherence.

The results of a recent feasibility study on the outpatient administration of cell therapies is creating growing interest in whether home-based management may be possible in the future.

In her review of the specialty drug pipeline, Evernorth's Aimee Tharaldson said upcoming approvals for Crohn’s and colitis drugs could further shift the drug spend from the medical benefit to the pharmacy one.
A pharmacist-led program to switch patients to biosimilars at the Mayo Clinic improved access and lowered costs, the clinic's Chelsee Jensen, Pharm.D., said during a presentation today.

Climate change and global travel have brought a reemergence in the United States of viral diseases spread by mosquitos.
The CDC has learned from the COVID-19 pandemic and is making changes to better identify and respond more effectiively to threats to public health.
Research has found that for patients with recurrent C. diff infections, improving the microbiome is helpful to patients’ physical, mental and social status.
Research of broadly neutralizing antibodies — which are able to neutralize multiple strains of HIV — have the potential to treat, prevent and maybe even cure HIV.

Yuflyma is on Ventegra’s public and private formulary on parity with the reference product Humira.

Inpefa is approved to reduce the risk of cardiovascular death and hospitalization for heart failure in adults. The wholesale acquisition cost is $598 per month.

Insurers and PBMs now have to follow a 2020 rule in which accumulator programs can only be applied to brand name drugs that have generic equivalents available.

Last week, a judge struck down a 2021 federal rule that allowed insurance companies to exclude pharmaceutical company copay assistance from annual deductibles.
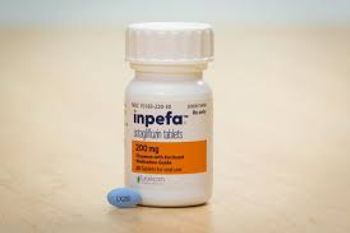
Inpefa is an inhibitor of both SGLT2 and SGLT1, approved to reduce the risk of cardiovascular death and hospitalization for heart failure in adults. The wholesale acquisition cost is $598 per month.
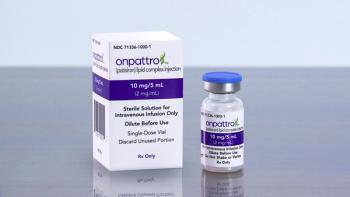
Alnylam Pharmaceuticals will no longer pursue this indication of Onpattro and will instead on focus on a label expansion for Amvuttra, which is in phase 3 development to treat patients with cardiomyopathy of ATTR amyloidosis.

The FDA approved the first biosimilar of the arthritis medication Actemra, as well as plaque psoriasis drug Zoryve for children. The agency issued a complete response letter for lebrikizumab in atopic dermatitis and for liquid formulation of botulinum toxin for frown lines, and assigned a review date for a gene therapy for a rare immune disorder. Additionally, Takeda plans to withdraw the oncology drug Exkivity from the market while Coherus resubmits BLA for Udenyca OnBody.

Mark Campbell, Pharm.D., RxBenefits’ senior vice president of clinical solutions, talks about how Medicare’s drug price negotiations could have a ripple effect on pharmaceutical company innovation and prices on the commercial side.