Study Finds Big Benefit From Approach to Newborns With Opioid Withdrawal Syndrome — ‘By Holding Them’
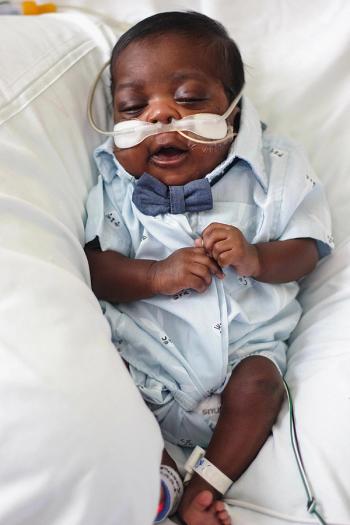
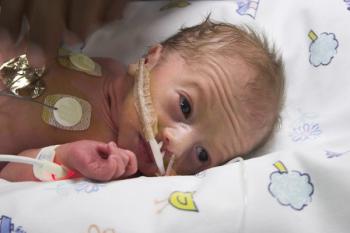
Most babies born to mothers who used opioids during pregnancy are delivered with a form of addiction called neonatal abstinence syndrome. It is treated in the hospital, but how long the treatment lasts depends on the severity of withdrawal symptoms called neonatal opioid withdrawal syndrome — the amount of distress that the newborn is experiencing.
The “Eat, Sleep, Console” approach for newborns with neonatal opioid withdrawal syndrome reduced average time in the hospital by 45% compared to usual care, which typically includes morphine, according to a randomized controlled study at 26 sites across the United States.
It was not a direct comparison but, rather, an analysis of time to discharge yielded by a standard withdrawal severity assessment tool, which the authors said may overestimate the need for pharmacologic treatment compared with this relatively new approach.
Although it was developed in 2014 and has experienced increasing use in recent years, whether it “can safely reduce the time until infants are medically ready for discharge when it is applied broadly across diverse sites is unknown," the authors wrote in the background section of the
“The Eat, Sleep, Console Care Tool relies on a function-based assessment of withdrawal severity that is focused on an infant’s ability to eat, sleep and be consoled, along with the use of nonpharmacologic interventions (e.g., low-stimulation environment, skin-to-skin contact, clustered care, and breast-feeding) as the first line of treatment and empowerment of families and caregivers in the care of their infants,” wrote first and corresponding author
They are caused by
NAS is successfully treated soon after birth, although infants experience extreme but temporary distress during withdrawal. But it results in none of the serious lifelong disabilities common to
For this study, Young and her colleagues enrolled 1,305 infants. They found that the number of days from birth until readiness for hospital discharge was 8.2 in the Eat, Sleep, Console group and 14.9 in the usual care group, a difference of 45%. Adverse outcomes were similar.
Related:
“The Eat, Sleep, Console approach” to babies born addicted to opioids, concludes an
Newsletter
Get the latest industry news, event updates, and more from Managed healthcare Executive.