
Results of this prospective cohort study fits with other evidence showing no association between COVID-19 vaccination and miscarriage.

Results of this prospective cohort study fits with other evidence showing no association between COVID-19 vaccination and miscarriage.

About 1,700 hospitals will receive lump sums after the Supreme Court ruled that CMS was wrong when it cut reimbursement rates for Part B drugs.
Nemolizumab quelled the itching caused by prurigo nodularis, a rare skin condition. By inhibiting interleukin-31, the experimental drug seems to have a downstream effect on other interleukins secreted during type 2 inflammation.

A subgroup analysis of real-world patient survey data found Black/African American patients with moderate to severe atopic dermatitis had improved disease control, symptoms and treatment satisfaction on Dupixent.

Delgocitinib is first-in-class pan-Janus kinase inhibitor

The announcement has triggered a wave of concerns among users of eye-care products, particularly in those designed to alleviate dry and irritated eyes.

Data from the study revealed that about half of the patients did not receive any treatment during their 12-month follow-up.

Recent data found that extreme heat is projected to lead to a significantly higher burden of excess cardiovascular deaths in the United States by midcentury (2036–2065), with elderly adults and non-Hispanic Black adults being most affected.

Per member spending in private insurance is expected to grow at 6.8% annually due to both utilization and increases in price.
Many carriers reported feelings of concern, anxiousness, and guilt for passing the X-linked inherited retinal disease to their children—and 78% of respondents in a new study believe that carriers should have access to gene therapy options.

The self-administered nasal spray may increase vaccination rates.
Capsid assembly modulators (CAM) work by assembling flawed hepatitis B capsids and preventing the formation of new infectious viruses.

The idea of software creating and delivering content to patients and plan members would have been unthinkable in the recent past. Now it is becoming essential to a good customer experience for patients.
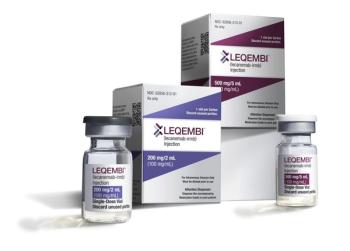
In new data, 60% of the patients with early stage Alzheimer’s disease and who have low levels of the protein tau achieved cognitive improvement when treated with Leqembi.

Managing Editor of Managed Healthcare Executive, Peter Wehrwein, had a discussion with William Shrank, M.D., a venture partner with Andreessen Horowitz, a venture capital firm in Menlo Park, California, about how artificial intelligence's role is improving healthcare, where we are today with value-based care and the ongoing efforts of reducing waste in the healthcare space for this episode of the "What's on Your Mind" podcast series.

The growth of digital twin technology — paired with the transition towards personalized medicine — has left many healthcare industry professionals evaluating the potential of a “patient twin.”

Results of a randomized trial reported in The Lancet Digital Health suggest a role for internet-based cognitive behavioral therapy, but many of the study volunteers were left with symptoms that would classify them as having major depressive disorder.
The drug has been engineered using a proprietary glycopegylated technology that is designed to increase its overall half-life.

CRISPR gene-editing therapy is predicted to cost about $1 million dollars per person which is considerably more expensive than keeping a patient on ART for the rest of their life.

Mental health disorders impact the lives of almost 1 in every 4 people who are living with HIV/AIDS here in the United States.

The Minnesota-based pharmacy benefit manager says a program that resulted in patients switching from two incretin therapy prescriptions to one yielded $7,500 in savings per patient and a total of $3.5 million.

In a platinum award winning poster presented at the AMCP Nexus 2023 conference in Orlando, “Specialty drug use varies by race and wage among employees with employer-sponsored health insurance,” authors expressed that spending on specialty medications for autoimmune conditions has increased in recent years, raising affordability concerns for employers.

The FDA’s decision to allow at-home dosing of intransal foralumab for patients with multiple sclerosis is likely to improve patient compliance to treatment and health outcomes, according to a recent release statement.

To add precision to the nomenclature and nosology and remove stigma, experts are moving to rename nonalcoholic fatty liver disease as metabolic dysfunction-associated steatotic liver disease.

Research continues to develop new therapies for rare cancers and to provide options that allow for fewer toxicities. At the same, however, more new drugs are launching with high price tags.

Adopting evolving computer system tools like artificial intelligence and machine learning in managed care pharmacies have resulted in efficiency when addressing the challenges they are faced with, according to Jessica Hatton, PharmD, BCACP, associate vice president of Pharmacy at CareSource and Nick Trego, PharmD, senior vice president of Clinical Analytics and Client Services at HealthPlan Data Solutions, Inc.

The program yielded savings of $25,000 per patient in its pilot phase but is not expected to produce savings as a routine offering because reimbursement for home infusion was matched to reimbursement at a facility. Horizon executive Timothy O’Shea, Pharm.D., M.S., says cost savings were a “secondary outcome” of the program and noted the high patient satisfaction.