
Public health policies and treatment advances have made a difference.

Public health policies and treatment advances have made a difference.

Radiation oncologists across the country met virtually with members of Congress last week to urge lawmakers to pass legislation that will safeguard access to high-quality, value-based healthcare for people with cancer.
Therapies that target lung cancer at the molecular level are proliferating — and so are the biomarkers for guiding their use.
In the early going, patients seeking the expensive, customized cancer treatments called CAR-T cell therapy had to go to an academic medical center. But that’s changing, as Duncan Allen, M.H.A., of the community cancer network OneOncology explains.

Two newly approved antibody-drug conjugates and changes in radiation therapy lead the way.

The Johns Hopkins professor and new MHE editorial advisory board member discusses screening among race, how certain screening tests intensify health disparities and how the Trump administration is not correctly applying science within healthcare in this final part of a four-part video series.

FDA approves new uses for companion diagnostics, while Janssen seeks a new indication for Xarelto.

Gilead's Veklury gets full approval; AstraZeneca's Tagrisso heads toward second indication in NSCLC; another use for experimental antifungal therapy.

The Johns Hopkins professor and new MHE editorial advisory board member lauds the effects of the Affordable Care Act but holds out for a program that “gets every human being the healthcare that every human being deserves,” in this second part of a four-part series.

This episode of Tuning Into The C-Suite welcomes our first of many episodes part of the new “Meet the Board” podcast series. Listeners will now hear from a member of Managed Healthcare Executive's Editorial Advisory Board once a month at the end of each month. The first guest featured is Physician and former Executive VP of the American Cancer Society, Otis Brawley. Brawley is a Bloomberg Distinguished Professor of Oncology and Epidemiology at Johns Hopkins University.

COVID-19 is certainly important. But oncologists, people with cancer, and complex ecosystem of cancer care in the U.S. are grappling with other important issues such as reimbursement, distorted incentives, the implications of the massive amount of data that is available, and, of course, high costs and prices. Included are thoughts from five experts on these challenges and how they might be met.

Financial Toxicity is a growing concern for many cancer patients and caregivers, and with the continued rise in treatment costs, it can no longer be ignored.

Actions from FDA this week include approval of a second targted treatment for a Duchenne muscular dystrophy mutation, and filings in COVID-19 and blood disorders.
Underrepresentation of blacks in clinical trials is garnering special attention.

The fast-evolving field of precision medicine offers the promise of more effective treatments and better outcomes, and it’s time that we collaborate to keep outdated clinical and reimbursement practices from slowing it down.

In this week's episode of Tuning Into The C-Suite podcast, MHE's Briana Contreras chatted with David Calabrese, R.Ph, MHP, who is senior vice president and chief pharmacy officer of pharmacy care services company, OptumRx. David is also a member of Managed Healthcare Executives’ Editorial Advisory Board. During the discussion, he shared the OptumRx Quarter 2 Drug Pipeline Insights Report of 2020. Some of the information shared includes the three notable drugs currently being reviewed or those that have been recently approved by the FDA. Also discussed were any interesting industry trends to watch for.
FDA approves Merck's Keytruda for certain patients with metastatic colorectal cancer a month after study results were presented at ASCO.
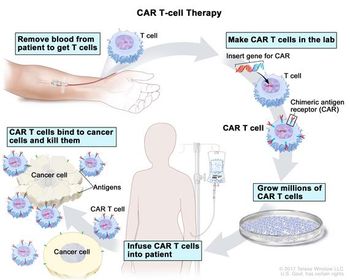
An off-the-shelf version of CAR-T therapy that uses the immune system’s natural killer cells is a possibility. But the high price of CAR-T shows some doubts about how widely it can be used.


During the American Society of Clinical Oncology 2020 Annual Meeting, Flatiron Health, Foundation Medicine, and Genentech presented plans for the Prospective Clinico-Genomic study (PCG), a low-interventional pilot that will use a technology-enabled prospective data collection platform to simplify data collection for patients with lung cancer being treated through clinical trials. The idea is collect blood samples using Foundation Medicine’s liquid biopsy assay and analyze the results through Flatiron’s platform, to see if genomic changes can be detected over the course of cancer treatment. Bobby Green, MD, chief medical officer for Flatiron Health, spoke with Managed Healthcare Executive®.




During the American Society of Clinical Oncology 2020 Annual Meeting, Flatiron Health, Foundation Medicine, and Genentech presented plans for the Prospective Clinico-Genomic study (PCG), a low-interventional pilot that will use a technology-enabled prospective data collection platform to simplify data collection for patients with lung cancer being treated through clinical trials.

Kathi Mooney, Ph.D., RN, FAAN, interim senior director of population sciences at the Huntsman Cancer Institute in Salt Lake City, discusses hospital-at-home programs and how they are common in countries with single-payer systems and include patients with conditions ranging from cellulitis to heart failure. The benefits include the familiarity of home surroundings, avoidance of hospital-acquired infection, and less de-conditioning from being in a hospital bed.