Findings from a mouse model suggest that encapsulating anandamide, an endocannabinoid, could be a way to make it an effective treatment for cutaneous lupus erythematosus (CLE).

Findings from a mouse model suggest that encapsulating anandamide, an endocannabinoid, could be a way to make it an effective treatment for cutaneous lupus erythematosus (CLE).

VISIBLE is focused on answering data gaps in people of color with psoriasis. Lead investigator Andrew F. Alexis, M.D., hopes the study will generate data to help address care gaps and inform future best practices in diversity research in dermatology.

The researchers say their findings argue for dermatologists embracing TikTok and promoting more high-quality content on it and other social media platforms.
Checkpoint inhibitors, such as Keytruda (pembrolizumab) and Opdivo (nivolumab), are playing a major role in cancer treatment. But they also produce side effects that affect the skin. Steven Chen, M.D., M.P.H., M.H.P. Ed., said dermatologists need to work with oncologists to manage the side effects so patients can stay on checkpoint inhibitors.

Longer-term use of Opzelura was well tolerated, with no serious treatment-related adverse events, according to a poster presented at the annual dermatology meeting.

Women who are pregnant don’t have to stop all of their treatments during pregnancy. Some can be safely treated for their psoriasis or eczema, according to a presentation today at the annual meeting of the American Academy of Dermatology.

Research shows that short-term exposure to wildfire air pollution can affect the skin and cause flares of certain skin conditions.
After initial treatment, Opzelura used on an as-needed basis was able to control symptoms of atopic dermatitis and helped patients sleep, according to a poster presented at the annual dermatology meeting.
Although not a common complication of hip, knee and other metal implants, the number of allergic reactions is growing, partly the number of joint replacements is growing, according to a presentation at the annual meeting of the American Academy of Dermatology. Nickel is the most common contact allergen.
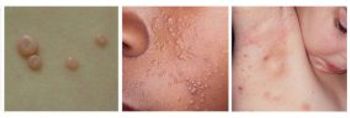
If approved, berdazimer could be the first prescription product treat molluscum, a common skin infection caused by poxvirus. The Prescription Drug User Fee Act goal date is Jan. 5, 2024.

Cibinqo is an oral, once-daily JAK1 inhibitor now approved for those 12 years of age and older.

In this Managed Healthcare Executive® K-Cast, David Rosmarin, M.D., vice chair of education and research in the Department of Dermatology at Tufts Medical Center in Boston, discussed the economic burden, treatment goals, treatment options and health insurance issues related to vitiligo.

Because vitiligo affects appearance, people with vitiligo are disproportionately affected by psychiatric conditions, including depression.
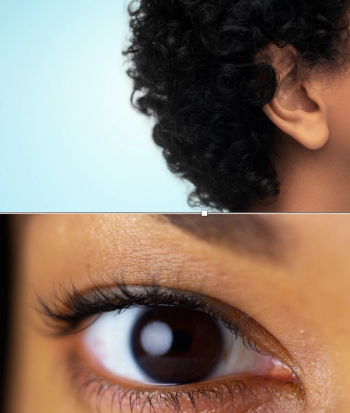
The melanocytes attacked by the autoimmune disease are present in the eyes and ears, not just the skin, so vitiligo can adversely affect vision and hearing.
Formulary placement is crucial to the commercial success of newly approved products, so Acrutis put out a press release last week announcing that Express Scripts had put Zoryve (roflumilast), the company's PDE4 inhibitor cream for plaque psoriasis, on its formulary.

Israeli researchers find more than a fivefold risk of developing systemic sclerosis among people with vitiligo.

Results from the test reveal that high levels of the immune biomarker Thymus and Activation-Regulated Chemokine (TARC) in babies increases their chances of developing atopic dermatitis.

Taking 80 mg of the high-cholesterol statin drug daily can control patients' vitiligo activity and protect them against the hazardous effects of dyslipidemia.
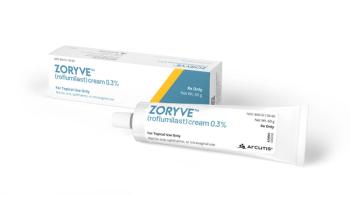
Launched in August, Zoryve is a once-daily, steroid-free cream and is the first topical PDE4 inhibitor approved to treat plaque psoriasis in patients 12 years of age or older.
Dupixent is the first treatment approved to treat prurigo nodularis, a chronic skin disorder characterized by the presence of hard, itchy nodules.
The search for the causes of vitiligo continues. The findings in this study stand in contrast to another one conducted in eastern China that showed a high percentage of children with vitiligo had thyroid abnormalities.
A recent review article lists six other JAK inhibitors that might be used as treatments for vitiligo in addition to ruxolitinib, the active ingredient in recently approved Opzelura.

While phototherapy has been shown to have a positive effect on vitiligo as it works to reduce or prevent the destruction of skin cells, treatment done in the hospital can be long-term and require a number of visits.

Commercial shipments are already under way of Cosette’s gel formulation of tazarotene to treat patients with acne and facial wrinkles.

Developed by Bristol Myers Squibb, Sotyktu is the first approved TYK2 inhibitor. It is expected to be available later this month.