
PBM reform has finally made its way through Congress and was signed into law by President Donald Trump. Here are six things you need to know about the law

PBM reform has finally made its way through Congress and was signed into law by President Donald Trump. Here are six things you need to know about the law

Cigna reported 11% revenue growth for 2025. Company executives focused on several key issues in its call with investors: its settlement with the Federal Trade Commission, a transformation announced last year to move to a rebate-free model in pharmacy benefits, and a focus on patient affordability.

Express Scripts agreed to overhaul its business model, ensuring patients pay the lowest available price for medications and reimbursing community pharmacies based on actual costs.

Rural hospital study found text messaging intervention significantly improved medication adherence for heart failure patients, demonstrating that simple technology can reduce readmissions.

Myqorzo was approved in December to treat adults with obstructive hypertrophic cardiomyopathy. It is available through a REMS program and has a boxed warning about the risk of heart failure.

Yuvezzi is the first combination product that treats adults with presbyopia. It will be available in the second quarter of 2026.

CMS announced 15 drugs for Medicare price negotiations under the IRA, representing $27 billion in spending and treating conditions such as diabetes, cancer, and autoimmune conditions. Here are three insights on the list from industry experts.
A new study has identified the brain connectivity patterns that lead to cognitive difficulties in patients with schizophrenia, suggesting potential targets for therapeutic intervention in early psychosis stages.

During an investor call, UnitedHealth Group executives talk about the steps it has taken to increase transparency, use technology to reshape its healthcare delivery and address how it adapting to financial pressures across its Medicare, Medicaid, and Affordable Care Act businesses.

In a Q&A, Jeffrey Hogan, managing director and co-founder of Judi Group, talks about the importance of identifying hidden costs and contract conflicts of healthcare vendors.

Legislation reforms pharmacy benefit managers by mandating price transparency, eliminating certain compensation models, and providing CMS funding to enforce contract terms.

Lawmakers from both parties grill insurance companies over claims denials, prior authorization delays, and vertical integration.

Judi Group brings together top benefits specialists, ERISA attorneys, actuarial analysts, and technologists to assess risk and protect fiduciary integrity across vendors and solutions.
Dynavax's Z-1018 shingles vaccine in a phase 1/2 trial showed comparable effectiveness to Shingrix with fewer injection site reactions in adults aged 50 to 69.

Medicare’s recently released drug pricing models GLOBE and GUARD use international price benchmarks to generate government rebates but experts say they are unlikely to reduce costs for patients.

Duravyu demonstrated vision improvement in phase 2 trials in patients with diabetic macular edema. Phase 3 trials are enrolling patients now.


Elevated levels of lipoprotein(a) is a genetic disease that causes cardiovascular disease. Steven Nissen, M.D., addresses why it’s important to find treatments for this genetic risk factor.

Beginning this month, PBMs in California must prioritize health plan interests over their own, and CVS Caremark’s CalPERS contract includes $250 million in performance guarantees.

Existing medications for narcolepsy aim for symptom control. But therapies in development are targeting the specific mechanism of the disease and the brain’s natural sleep-wake cycle.
Five experimental drugs show promise in significantly lowering lipoprotein(a). The furthest along is pelacarsen, with phase 3 results expected in the first half of this year, and regulatory filings in the second half of this year.
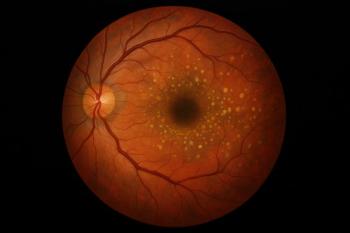
Lytenava, an ophthalmic bevacizumab formulation to treat patients with wet age-related macular degeneration, received yet another FDA complete response letter. The agency is asking for confirmatory efficacy data beyond existing NORSE trial results.

University of Florida researchers found immune systems destroy single beta cells before attacking larger pancreatic islets, explaining Type 1 diabetes progression and why younger patients lose cells more aggressively.

To gain approvals, biosimilar manufacturers could do faster, less expensive analytical studies of their products' composition.

The results of an American Society of Health-System Pharmacists survey shed some light on what is on the minds of pharmacy leaders at health systems.
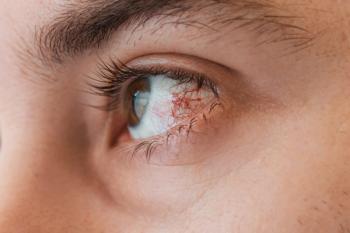
Ophthalmologists and oncologists created a new ocular adverse event grading scale for oncology trials that addresses ambiguities and provides clear dose modification recommendations.

The biosimilar market experienced 16 new approvals by early December 2025, marking significant expansion and introducing previously unavailable biosimilar drug classes to patients.

Medicare's first negotiated drug prices, effective January 2026, provide modest discounts, with limited savings for most beneficiaries and some unintended consequences.

A new Tufts study found that Medicare price negotiations on biologics with imminent biosimilars could lead to near-term savings, but greater savings are likely from biosimilar competition.
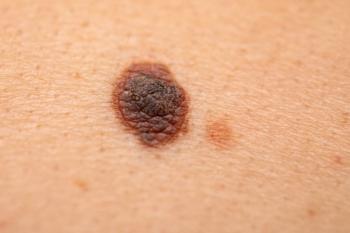
Melanoma survival rates have doubled since 2009. New treatments, including oncolytic viruses, fecal transplants, and engineered antibodies, have shown promising results in clinical trials.
Published: August 7th 2025 | Updated: August 14th 2025
Published: August 12th 2025 | Updated: August 14th 2025
Published: September 28th 2021 | Updated:

Published: August 28th 2023 | Updated:

Published: September 20th 2023 | Updated:
Published: July 20th 2021 | Updated: